Osmosis is the movement of water molecules across a selectively-permeable membrane down a water potential gradient. It is a physical process in which a solvent moves, without input of energy, across a semipermeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis releases energy, and can be made to do work.
Osmosis is important in biological systems, as many biological membranes are semipermeable. In general, these membranes are impermeable to organic solutes with large molecules. Permeability may depend on solubility properties, charge, or chemistry, as well as solute size. Osmosis provides the primary means by which water is transported into and out of cells.
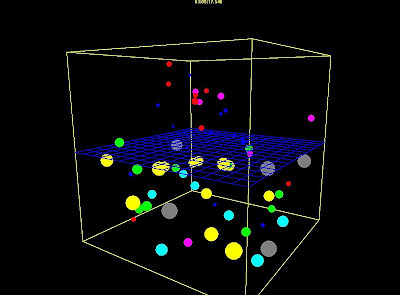 |
| Shot of a computer simulation of the process of osmosis |
No comments:
Post a Comment